Overview of Hexose monophosphate shunt pathway
The Hexose Monophosphate Shunt (HMP) Pathway, also known as the pentose phosphate pathway (PPP), phosphogluconate pathway, or the pentose shunt, serves as an alternative route for glucose oxidation. Unlike glycolysis, the HMP pathway does not directly consume or produce ATP. Instead, it is primarily focused on generating reduced power in the form of NADPH.
Location: Present in various tissues, including the liver, lactating mammary glands, adipose tissue, adrenal cortex, and red blood cells.
Why Occurs in cell cytoplasm?
The Hexose Monophosphate Shunt (HMS) Pathway provides a mechanism for oxidizing glucose to produce NADPH, which is crucial for the biosynthesis of various biomolecules, particularly fats.
Additionally, the Hexose Monophosphate Shunt Pathway (HMP) can be utilized for the breakdown of dietary pentose sugars, the production of pentose sugars for nucleotide synthesis, and the metabolism of less common four- and seven-carbon sugars.
Why the Biological Importance of HMP?
-
NADPH produced in the HMP shunt is utilized for the biosynthesis of several important factors in various organs.
-
In the liver, NADPH is utilized for fatty acid synthesis, cholesterol synthesis, bile acid synthesis, glutamate synthesis, and the cytochrome P450-hydroxylase system in the body.
-
In the adrenal cortex and gonads, NADPH is utilized for cholesterol and hormone synthesis.
-
In the adipose tissue, NADPH is utilized for fatty acid synthesis.
-
NADPH is utilized for the formation of deoxyribonucleotides and pyrimidine nucleotides in the body.
-
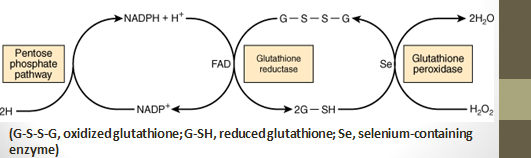
- In RBCs, NADPH produced is utilized for the formation of reduced glutathione from oxidized glutathione. Glutathione reductase catalyzes this reaction.
-
Reduced glutathione is required for the removal of hydrogen peroxide (H2O2) by glutathione peroxidase for the conversion of methemoglobin to normal hemoglobin and the improvement of –SH groups of erythrocyte proteins.
-
Cells with lowered glutathione levels are more prone to hemolysis.
-
- Pentoses produced in this pathway are utilized for nucleic acid synthesis & nucleotide coenzymes like NAD+, FAD, and FMN synthesis.
- A non-oxidative phase of this pathway turns pentoses of endogenous or dietary nucleic acids into intermediates of glycolysis where they are further oxidized to generate energy.
- Interconversion of three, four, five, six, and seven carbon sugar compounds in the non-oxidative phase metabolically connects these sugars to the glycolysis pathway.
Tissues in which the Pentose Phosphate Pathway is Active:
- High Activity occurs in the liver, adipose tissue, adrenal cortex, thyroid, erythrocytes, testis, and lactating mammary gland.
- These tissues use NADPH in the reductive pathway, for example, fatty acids, steroids, and amino acids.
- Low Activity occurs in skeletal muscle and non-lactating mammary glands.
Phases of HMP Shunt
This pathway occurs in the following two phases:
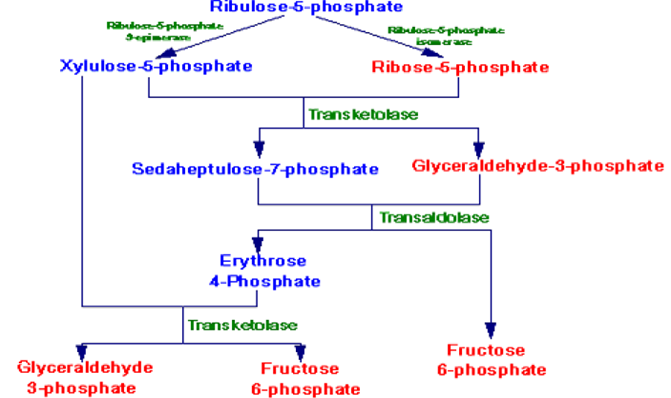
- Oxidative Phase: Glucose-6-phosphate is oxidized to ribulose-5-phosphate, producing NADPH and CO2.
- Non-oxidative Phase: Interconversion of pentose phosphates and glycolytic intermediates.

- NADPH Production:
- In the oxidative first phase of the HMP pathway, glucose 6-phosphate is oxidatively decarboxylated to a pentose sugar, ribulose 5-phosphate.
- The first enzyme of this HMP pathway, glucose 6-phosphate dehydrogenase, oxidizes the aldehyde at C1 & reduces NADP molecule to NADPH molecule.
- Thus, 2 moles of NADPH per mole of glucose 6-phosphate are formed from this part of the pathway.
- Glucose-6-P is a branch site in carbohydrate metabolism.
- It is a precursor for almost every pathway that utilizes glucose, including the glycolysis cycle, the pentose phosphate pathway, and glycogen synthesis.
- From the opposite perspective, it also can be generated from other pathways of carbohydrate metabolism in the body, such as glycogenolysis (the breakdown of glycogen compound), the pentose phosphate pathway, and gluconeogenesis (the synthesis of glucose from non-carbohydrate compounds).
- Ribose 5-phosphate from the oxidative branch of this pathway:
- To generate or produce ribose 5-phosphate from the oxidative pathway, the ribulose 5-phosphate formed from the action of the 2 oxidative steps which is isomerized to produce ribose 5-phosphate (a ketose-to-aldose transformation, similar to fructose 6-phosphate being isomerized to glucose 6-phosphate).
- The ribose 5-phosphate can then penetrate the pathway for nucleotide synthesis, if required, or can be converted to glycolytic intermediate compounds, as explained below for the non-oxidative phase of the HMP.
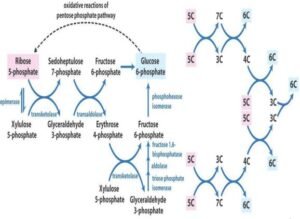
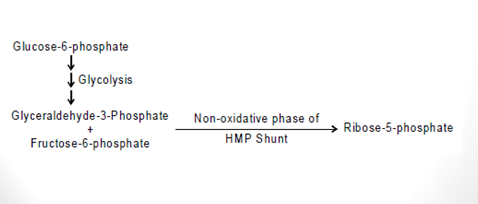
The Non-oxidative Phase HMP
- The non-oxidative reactions of this pathway are reversible reactions that permit intermediates of the glycolysis pathway (specifically glyceraldehyde-3-P and fructose-6-P) to be converted to 5-carbon sugars (such as ribose-5-P) and vice versa in this pathway. The needs of the cell will dictate in which direction this pathway proceeds.

Regulation of HMP shunt:
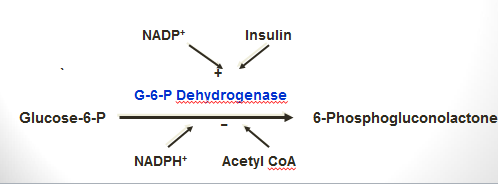
- G-6-phosphate dehydrogenase is the rate-limiting enzyme of HMP-shunt.
- It is stimulated by insulin molecules & NADP+.
- It is inhibited by NADPH, H+, and Acetyl CoA molecules.
Control of the HMP Shunt:
- The major control of the pathway is exerted at the first step, which is the glucose-6-phosphate dehydrogenase reaction. Features include:
- This is an essentially irreversible reaction.
- The major controlling factor is the ratio of NADPH to NADP+.
- As the cell utilizes NADPH, the concentration of NADP+ increases. This activates the pathway, increasing NADPH generation to compensate.
- Therefore, the pentose phosphate pathway is operated by a low NADPH: NADP+ ratio.
- The control of the non-oxidative phase is by the essential products, namely ribose-5-phosphate and NADPH.
- The individual needs of the cell for either of these decide whether the production of ribose-5-phosphate or fructose-6-phosphate and glyceraldehyde-3-phosphate predominates. For example:
- If the NADPH requirements are considerable, all the ribose-5-phosphate formed is converted to fructose-6-phosphate and glyceraldehyde-3-phosphate. These are converted back to glucose-6-phosphate to enter the pentose phosphate pathway again and generate more NADPH.
- When numerous pentoses are needed, glucose-6-phosphate is converted to fructose-6-phosphate and glyceraldehyde-3-phosphate by glycolysis. Pentoses are formed from these molecules through the non-oxidative phase of the pathway without NADPH production.

Differences between HMP shunt and glycolysis:
| Glycolysis | HMP pathway | |
| In all cells | In certain cells | Location |
| Phosphorylation occurs 1st then oxidation | Occurs in the 1st reaction | Oxidation of glucose |
| NAD+ | NADP+ | Coenzyme |
| 2 or 8 ATP | No energy production | Energy |
| Not produced | Produced | CO2 |
| Not produced | Produced | Pentoses |
HMP Shunt & Glutathione Peroxidase Protect Erythrocytes against Hemolysis:
- In red blood cells (RBCs), the Hexose Monophosphate Shunt (HMP) pathway provides NADPH for the reduction of oxidized glutathione.
- Reduced glutathione removes hydrogen peroxide (H2O2).
- Accumulation of hydrogen peroxide (H2O2) may decrease the lifespan of RBCs by causing oxidative damage to the cell membrane, leading to hemolysis.
Medical Importance of HMP Shunt
- Glucose-6-phosphate dehydrogenase deficiency: Some individuals have a 10-fold decrease in active glucose-6-phosphate dehydrogenase due to sex-linked defective genes.
- The less active glucose-6-phosphate dehydrogenase becomes inactive in the presence of certain drugs. Affected individuals are normal until exposed to these drugs.
- Glucose-6-phosphate dehydrogenase deficiency occurs when drugs like aspirin, primaquine anti-malarial drug, and sulfonamide are administered.
- NADPH secretion is blocked in these individuals due to the deficiency of glucose-6-phosphate dehydrogenase, increasing the susceptibility of RBCs to hemolysis.
- The affected individuals have a chance to develop hemolytic anemia on exposure to these drugs.
- Consumption of fava beans also causes glucose-6-phosphate dehydrogenase deficiency in susceptible individuals, known as favism.
- Transketolase deficiency can occur in cases of thiamine deficiency.
- Wernicke-Korsakoff encephalopathy: This condition is due to defective genes.
- Transketolase of affected individuals has a lower affinity for TPP.
- Characteristic symptoms include abnormal walking and standing, memory loss, and paralysis of eye movements.
- The disease is demonstrated only when there is a thiamine deficiency.
[embeddoc url=”https://notesmed.com/wp-content/uploads/2020/08/HMP-SHUNT.pdf” download=”all” cache=”off”]