What is the citric acid cycle?
The citric acid cycle or TCA cycle is the last common pathway for the oxidation of different fuel molecules amino acids, fatty acids, and carbohydrates. Most different fuel molecules enter the TCA cycle as acetyl coenzyme A.
A cyclical series of 8 reactions that oxidizes one molecule of acetyl CoA entirely to two molecules of carbon dioxide (CO2), generating energy, either directly as ATP or in the form of reducing equivalents such as NADH or FADH2.
The cycle is aerobic; the absence or deficiency of oxygen leads to total or partial inhibition of the TCA cycle.
Location: All mammalian cells that contain mitochondria (i.e. not in red blood cells)
Site: All the enzymes of the citric acid cycle or TCA cycle are located in the mitochondrial matrix.
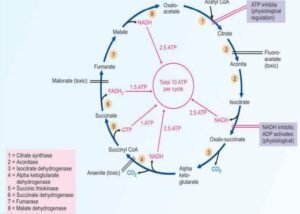
Functions of Citric acid cycle or TCA cycle:
The functions of the citric acid cycle or TCA cycle are the following listed below;
- The citric acid cycle or TCA cycle provides a final or last common pathway for the oxidation of carbohydrates molecules, fat, and protein compounds.
- The main function of this cycle is the production of energy, either, directly generating ATP or reducing equivalents such as NADH or FADH2, which are oxidized by the electron transport chain (ETC) in the body.
- The cycle provides substrates for the electron transport chain (ETC).
- The cycle is also a source or creator of biosynthetic precursors, for example, porphyrin which is synthesized from succinyl CoA, & amino acids are synthesized from oxaloacetate compound & α- ketoglutarate.
- Some of the cycle intermediates also exert regulatory effects on other pathways; for example, citrate inhibits phosphofructokinase (PFK-1) in glycolysis.
Reactions of the citric acid cycle:
The oxidative decarboxylation of pyruvate to acetyl CoA via. pyruvate dehydrogenase enzyme complex.
- Formation of citrate:
- Krebs’s cycle properly begins with the condensation of acetyl CoA & oxaloacetate, which is catalyzed by the citrate synthase enzyme in the cycle.
- Citrate is isomerized to isocitrate:
- It is done by enzyme aconitase. It is achieved in a two-stage reaction of dehydration followed by hydration through the formation of an intermediate compound is cis-aconitase.
- Formation of a-ketoglutarate:
- The involved enzyme is isocitrate dehydrogenase (ICD) that catalyzes the conversion (oxidative decarboxylation) of isocitrate to oxalosuccinate and then to α-ketoglutarate.
- The formation of NADH and the liberation of carbon dioxide (CO2) occur at this stage in the cycle.
- Conversion of a-ketoglutarate to succinyl CoA:
- It occurs through oxidative decarboxylation & catalyzed by the α -ketoglutarate dehydrogenase enzyme complex.
- The enzymes are dependent on cofactors-TPP, lipoamide, NAD+, FAD, and CoA.
- The mechanism of the reaction is analogous to the conversion of pyruvate to acetyl CoA. At this stage, the second NADH is produced and the second carbon dioxide (CO2) is liberated.
- Formation of succinate:
- Succinyl CoA is changed into the succinate by enzymes succinate thiokinase.
- This reaction is combined with the phosphorylation of GDP to GTP. This is substrate-level phosphorylation. Guanosine-5′-triphosphate (GTP) changes to ATP by the nucleoside diphosphate kinase enzyme.
- GTP + ADP→ATP + GDP
- Conversion of succinate to fumarate:
- This conversion reaction occurred by the succinate dehydrogenase enzyme.
- This reaction results in the production or secretion of FADH2 but not NADH.
- Formation of malate:
- The enzyme fumarase catalyzes the fumarate that converts into the malate with the addition of water (H2O).
- Conversion of malate to oxaloacetate:
- This reaction occurred by enzyme malate dehydrogenase and the third and final synthesis of NADH occurs in this stage.
- The oxaloacetate element is regenerated which can merge with another molecule of acetyl CoA, and again continue the cycle.
Summary of this cycle:
Acetyl CoA + 3 NAD+ + FAD + CDP + Pi + 2H2O →2CO2 + 3H+ + GTP + 3NADH + FADH2 + CoA.
Generalizations on the Regulation of Metabolic Pathways:
- The regulation of metabolic pathways takes place at rate-limiting steps, the slowest steps, in the pathway. These are reactions in which a little change of rate will affect the flux through the entire pathway.
- The regulation generally occurs at the 1st committed step of a pathway or at metabolic branch points. In human cells, most pathways are interconnected with other different pathways & have regulatory enzymes for every branch point.
- The regulatory enzymes often catalyze physiologically irreversible reactions. These are also the steps that vary in biosynthetic & degradative pathways.
- Numerous pathways have a “feedback” regulation system, that is, the end product of the pathway controls the rate of its own synthesis. A feedback regulation may include or involve the inhibition of an early step in this pathway (feedback inhibition) or regulation of gene transcription.
- Human cells use compartmentation to control the entrance of substrate & activators or inhibitors to different enzymes in the cycle.
- Hormonal regulation integrates responses in pathways involving more than one tissue. Hormones generally regulate fuel metabolism by:
- Changing the phosphorylation state of enzymes.
- Changing the quantity of enzyme present by altering its rate of synthesis (often induction or repression of mRNA synthesis) or degradation.
- Changing the concentration of an activator or inhibitor.
Regulation of TCA cycle:
- Citrate synthase:
- Citrate synthase enzyme, which is the 1st enzyme of the citric acid cycle or TCA cycle, is a simple enzyme that has no allosteric regulators.
- Citrate is a competitive inhibitor or resistance of oxaloacetate for citrate synthase (product inhibition); the fall in [citrate] caused by increased or intensified isocitrate dehydrogenase activity increases the rate of citrate formation.
- Succinyl-CoA also engaged with acetyl-CoA in the citrate synthase reaction in the cycle (competitive feedback inhibition).
- Isocitrate dehydrogenase is considered one of the rate-limiting steps of the citric acid cycle or TCA cycle and is allosterically activated by ADP and inhibited by NADH.
- The α -ketoglutarate dehydrogenase enzyme complex, even though not an allosteric enzyme, is product-inhibited by NADH and succinyl CoA, and may also be inhibited by GTP.
Thus, both α-ketoglutarate dehydrogenase enzyme & isocitrate dehydrogenase enzyme respond directly to alters in the relative levels of ADP and hence the rate at which NADH is oxidized by electron transport.
Both of these enzymes are also activated or regulated by Ca2+. In contracting heart muscle, & maybe other muscle tissues, the release of Ca2+ from the sarcoplasmic reticulum in the course of muscle contraction may provide an additional activation of these enzymes when ATP is being rapidly hydrolyzed.
Calcium (Ca2+) as a regulator:
Ca2+ among its numerous biological functions is an essential metabolic regulator. It stimulates or regulates glycogen breakdown, triggers muscle contraction, & mediates numerous hormonal signals as a second messenger.
Ca2+ also plays an important role in the regulation of the TCA cycle. It activates or regulates pyruvate dehydrogenase phosphatase enzyme & inhibits pyruvate dehydrogenase kinase enzyme, thereby activating the PDC to produce acetyl-CoA.
In addition, Ca2+ activates both the isocitrate dehydrogenase enzyme & the α -ketoglutarate dehydrogenase enzyme. Thus, the same signal stimulates muscle contraction & the production of ATP molecules to fuel it.
Respiratory control:
This is governed (controlled) by the activity of the electron transport chain (ETC) that oxidizes NADH & FADH2 and the rate of oxidative phosphorylation (ATP synthesis).
The activity of the TCA cycle is depending on the continuous supply of NAD+ and FAD, a cofactor for dehydrogenases.
The ETC is responsible for oxidizing any NADH and FADH2 formed during glycolysis and citric acid cycle or TCA cycle back to their oxidized forms, i.e. NAD+ and FAD.
Therefore, anything affecting the supply of substrates, namely oxygen (O2), ADP, or the source of reducing equivalents, may inhibit the cycle.
Inhibitors of Krebs cycle:
| Enzyme | Inhibitor |
| Aconitase | Fluoroacetate (non-competitive) |
| α-Ketoglutarate dehydrogenase | Arsenite (non-competitive) |
| Succinate dehydrogenase | Malonate (competitive) |
[embeddoc url=”https://notesmed.com/wp-content/uploads/2020/08/TCA-Cycle.pdf” download=”all”]